Parkinson's DiseaseSince the mid-1990s, Deep Brain Stimulation Has Been Used to Decrease Motor SymptomsReviewed by James FitzGerald, PhD FRCS(SN)
In the case of “John” (whose name and minor details have been altered), the onset began with a slight tremor in his left arm. Then his family noticed that his left leg would drag when he walked. Looking back, attentive family members recalled his left arm also didn’t swing much when he walked. Over the next year or so, these symptoms gradually worsened and spread. Eventually, the stiffness and slowness severely interfered with even the most mundane activities, such as dressing or walking. After consultation with a neurologist, John’s Parkinson’s disease was diagnosed. Loss of Dopamine, Movement ProblemsHe was far from alone; Parkinson’s disease is the second most common neurodegenerative disease. A report in 2017 estimated that it affects over 6 million people worldwide. The main symptoms include stiffness (also known as rigidity), slowness of movement (also known as bradykinesia), problems with gait (freezing or shuffling), instability which may lead to falls, and tremor. Patients often report periods of severe symptoms ('off' periods) in between periods of relief when movements are more normal ('on' periods). Parkinson’s is caused by progressive loss of brain cells (neurons) in a small area of the brain known as the substantia nigra. These cells produce a chemical called dopamine which is important to the normal functioning of brain circuits that control movement. Depletion of dopamine disrupts normal function of these circuits, resulting in the symptoms described above. Dopamine replacement, in the form of a medication called levodopa, is the main medical therapy for Parkinson’s. Initially the symptoms respond well to medication, but after several years of treatment patients often develop more frequent and unpredictable 'off' periods, and unwanted and troublesome movements called dyskinesias may appear during 'on' periods. It becomes progressively harder to find a good level of medication that is enough to prevent the patient being 'off' but without causing dyskinesias. Unfortunately, even with medication providing relief of initial symptoms for patients such as John, Parkinson’s disease remains progressive, and there is currently no therapy that has been proven to cure or slow the progression of disease. It is estimated that 28% of Parkinson’s patients suffer from debilitating motor symptoms despite optimal medical therapy. For many of these patients, surgical intervention can help restore the fluidity of movement that we all take for granted. Like a Pacemaker for the BrainDeep brain stimulation (DBS) is a surgical treatment sometimes used in Parkinson’s and other conditions. In this treatment, small pulses of electrical current are applied to specific locations in the brain through implanted electrodes. These electrodes are connected by wires that run under the skin to a programmable “internal pulse generator”, which is usually implanted just under the collarbone (sometimes referred to as an "implantable pulse generator"). It contains a battery and some electronics to generate the pulses, similar to a heart pacemaker. The device delivers electrical stimulation to specific brain areas that are involved in movement control. Electrical stimulation at precise locations in the brain is thought to restore the balance of the circuits that are disrupted in Parkinson’s disease. DBS can alleviate tremor, reduce stiffness, and lessen dyskinesias. It can very effectively smooth out on/off fluctuations. Prior to the introduction of DBS in the 1990s, the main surgical treatment for Parkinson’s disease involved inserting a probe into the brain and heating the tip of the probe to burn a very small region of brain tissue. The burn, known as a ‘lesion’, was made at the same targets that we now stimulate. Lesional surgery is still a useful option in some cases where DBS is not possible. Recently it has become possible to create lesions noninvasively using focused ultrasound. In fact, DBS evolved from lesional surgery. During lesioning procedures, it was observed that high-frequency stimulation at the target produced clinical effects similar to the lesioning itself. Stimulation via the inserted probe was routinely used to confirm successful targeting prior to burning. Technology subsequently advanced to allow such stimulation to be delivered continuously by an implantable device. Screening Patients for Surgical TreatmentImplanting electrodes in the brain carries risks, and before committing to surgery patients are assessed carefully to ensure that in each case the likely benefits from surgery outweigh the chance of harm. The assessment process will normally involve a neurosurgeon, a neurologist, and often a psychologist. It used to be believed that DBS should be offered once patients had reached the stage when medication was no longer working. However it is now clear that this is too late, and patients should not wait until their symptoms have advanced so far that they are severely debilitated all the time, even on maximum medication. In fact, responsiveness to levodopa prior to operation has been shown to be the best predictor of DBS efficacy. DBS should be considered once there are motor symptoms severe enough to significantly compromise function and quality of life, including on/off fluctuations, dyskinesias, or functionally impairing tremor. In order to assess responsiveness to levodopa it is usual to stop the patient's Parkinson's medication for a short time so that the physician can see how they are without it, then restart it and evaluate the difference it makes. Generally, a patient should have had symptoms for at least three or four years, to allow time to rule out the presence of atypical Parkinson syndromes, since DBS is not recommended for patients with these conditions. DBS is also not recommended in cases of dementia, inadequately treated psychiatric illness, extensive brain atrophy, or if the patient has medical conditions which mean they are not fit enough to undergo surgery under general anesthesia. Patients should consult their neurosurgeon prior to DBS implantation if they anticipate needing future MRI scans of the body, because some DBS systems are not compatible with MRI scanning. ComplicationsComplications of DBS surgery may include bleeding within the brain, hardware malfunction, skin erosion, and infection. Bleeding in a patient’s brain has been reported to occur in less than 3% of electrode placements, and to cause permanent deficits in less than 1% of cases. Infection rates have been reported to be less than 5%, similar to other neurosurgical procedures. Infection usually requires hardware removal, as well as antibiotic therapy. Stimulation can sometimes cause side effects due to the stimulus pulses affecting brain areas near to the intended target. However these effects are reversible if the stimulation is turned off, and the stimulation parameters can be adjusted very flexibly in order to maintain benefits while avoiding side effects. To objectively track the efficacy of DBS, most centers perform preoperative and serial postoperative clinical evaluations using motor testing scores, as well as obtaining quality of life and neuropsychological scores. Planning and Surgical TechniquePrior to surgery the operation is planned on a computer using MRI scans. The surgeon chooses ‘targets’ in the brain (i.e. the locations where the electrodes need to be implanted). Usually two electrodes are inserted, one on each side of the head. After selecting each target point, an entry point is chosen where a hole will be drilled in the skull to pass the wire through. The entry point is chosen such that the trajectory path avoids blood vessels, thereby reducing the incidence of bleeding complications. The brain targets are very small and electrodes must be implanted very precisely, to within a millimeter or so of the ideal location. This is usually performed using a rigid metal frame (known as a stereotactic frame) attached to the patient’s head to guide the drilling of the hole and the trajectory of the lead as it is implanted. There have also been developments of frameless systems, some of them involving the use of surgical robots, with equivalent accuracy and improved patient comfort.When DBS was introduced it was impossible to see the targets directly on the brain scans that were available at the time. Brain atlases were available giving coordinates for the targets within the ‘average’ brain, but each person’s brain is a slightly different shape and size, so this initial plan is only an approximation. Given these limitations confirmation of target localization in the operating room during surgery played a critical role in DBS implantation. Target confirmation may be performed by recording signals from brain cells in the target region using microelectrodes (fine wires much narrower than the DBS electrode itself) before implanting the stimulator electrode, or by doing test stimulation through the implanted electrode, or both. This part of the DBS implantation process is done with the patient awake so the surgical team can evaluate the effects of stimulation on the patient’s symptoms and check for any side effects. These time-honored methods are still in use in many centers worldwide. However the techniques used in some centers to implant DBS electrodes into the brain have changed considerably in recent years, because the MRI scans that are used to plan the operation nowadays are of far greater quality than was available when DBS was introduced. In particular, the boundaries of the subthalamic nucleus and the globus pallidus internus, which are the most commonly used targets, can now be clearly seen on modern imaging. Some surgeons have therefore stopped using microelectrode recording and now perform the entire implantation procedure under general anesthesia.
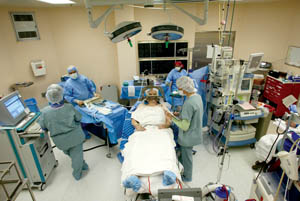
Caption: The Sacramento, Calif. operating room where Parkinson's disease patient Joel Davis undergoes his deep brain stimulation brain surgery for Parkinson’s disease - photo (2004) reproduced with permission of the photographer Implanting the NeurostimulatorAfter John underwent preoperative testing, his physicians informed him he would be a good candidate for DBS. He had already weighed its risks and benefits. On the day of surgery, he was brought into the operating room and given sedatives while his surgeon created two holes in his skull (one on each side) at the pre-planned entry sites and mounted an apparatus that would guide the test electrodes along the pre-planned trajectories. Then he was allowed to wake up fully before the microelectrodes were slowly advanced to the planned target in his brain. The electrode was then connected to an extension wire, which was tunneled under the skin down the neck and connected to the internal pulse generator implanted over the chest wall, much like a cardiac pacemaker. (Some centers do this part of surgery on a different day.) Altogether, implantation takes several hours.
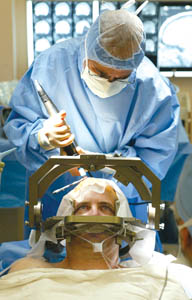
Caption: Dr. Conrad Pappas of Kaiser Permanente in Sacramento, Calif. performs deep brain stimulation surgery on Parkinson’s disease patient Joel Davis - photo (2004) reproduced with permission of the photographer Finding Maximum Relief from SymptomsA few weeks after his DBS system was implanted, John underwent his initial session to turn it on and test different settings to maximize relief of his symptoms. While DBS is not a cure, and does not stop the condition progressing, it can significantly improve patients’ quality of life, and has become an important part of the treatment of Parkinson’s disease to improve patients’ movement control. DBS has become the standard of surgical care for appropriately chosen patients like John, extending the time they can experience relief from disease symptoms. As the use of DBS becomes more widespread, physicians are gaining a better understanding of its efficacy and limitations. Newer targets in the brain are being investigated to capture the symptoms still inadequately controlled by current methods. Please note: This information should not be used as a substitute for medical treatment and advice. Always consult a medical professional about any health-related questions or concerns. Further reading1. Lang AE. When and how should treatment be started in Parkinson disease? Neurology . 2009;72(7 Suppl):S39-43. Resources
WIKISTIM – This free-to-use collaborative, searchable wiki of published primary neuromodulation therapy research was created in 2013 as a resource for the global neuromodulation community to extend the utility of published clinical research. The goals of WIKISTIM are to improve patient care and the quality of research reports, foster education and communication, reveal research needs, and support the practice of evidence–based medicine. December 9, 2018 |
| Last Updated on Friday, December 17, 2021 06:52 PM |